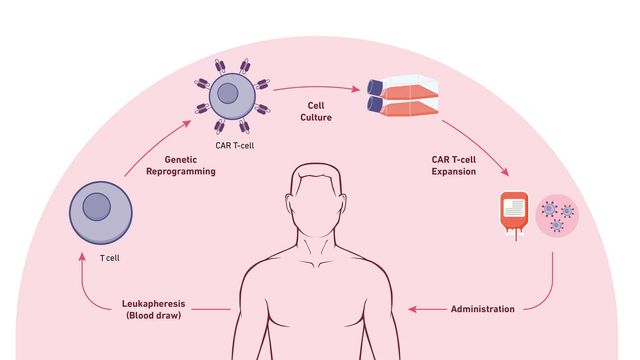
Cell Therapies on Ice: Seven Trends in Cryopreservation To Enable Cell and Gene Therapy Research
Medical and scientific advances have brought us incredible opportunities, from providing new cures to improving the quality of life for various conditions. These advances have been powered, in part, by cryopreservation, which enables researchers to preserve the functionality of specimens for extremely long periods of time.
Cryopreservation has a place in many applications, from CAR T-cell therapies fighting cancer to assisted human reproduction helping create the next generation of families. Given the importance of all these activities, it is critical to understand how improvements in cryopreservation can support and enhance efforts to bring these advances to the people who need them most.
In this listicle, we’ll explore the prevailing trends shaping advanced cryopreservation practices, including seven key trends that underpin effective cryopreservation and biosample management as an essential element in supporting research and cell and gene therapy.
1. Keep up with clinical evolution
The landscape is evolving towards enhanced clinical access, necessitating tailored solutions for clinical trials. As research labs manage clinical trials through each phase, more and more people are involved, which is why scalability should be an important consideration from day one. As the number of samples increases, so does the complexity of carefully handling cryopreserved samples, as well as managing and tracking them. For example, if a patient decides to withdraw from a trial, the researcher needs to know how to access their individual sample and remove it, without jeopardizing the other cryopreserved samples.
2. Leverage automation advancements
Automation in cryopreservation eliminates the human error factor. It is becoming pivotal in streamlining cryostorage processes, bolstering efficiency and ensuring sample integrity. An automated system handles sample management and supports compliance – it knows where each sample is and delivers it, without the manual process of digging through freezers. This helps keep lab workers safe by keeping them out of the liquid nitrogen tanks, along with ensuring the safety of the samples they manage. As a lab scales from 100 samples in storage to a million, automation makes it much easier for researchers to find what they need. Organizations are increasingly opting for hybrid methods of cryopreservation storage (manual and automated) as well as hybrid locations of storage (onsite and offsite, internally and externally managed, etc.) depending on the level of research activity and value of the samples.
3. Manage cold chain complexity
During the pandemic, everyday consumers became well-versed in supply chain complexities. Similarly, the cold chain presents challenges where cryopreservation processes can break down. The cold chain encompasses everything that happens to a sample from the moment it's frozen down, through the storage process, until the moment it's thawed and administered to a person or used in a research process. This includes any transit between sites, which can be difficult to account for when third-party logistics carriers like UPS or FedEx take custody of the sample. Effectively managing the cold chain warrants meticulous oversight and tracking of temperature data for end-to-end traceability.
4. Increase sample viability with precision tracking
Innovative tracking mechanisms are crucial to monitor access and exposure, ensuring sample viability and supporting regulatory compliance. When the stakes are curing cancer or other life-altering diseases, there's no room for error. If a cryopreserved cell is damaged, that therapy won’t be as effective for the patient. Additionally, with the costs of CAR T therapies upwards of half a million dollars or more, precision tracking and higher sample viability can help boost the efficiency of the entire process and lower costs per viable sample. Manufacturers of these therapies are demanding tracking and traceability to show precisely where the sample is, the temperature it is being held at, and the history of what has happened with each sample. Automation in the cryopreservation process also delivers increased security with administrative-defined user level controls and access.
5. Minimize cell damage caused by transient warming
Transient warming can happen any time a sample comes out of the cryogenic freezer, and this can reduce cell recovery. When freezer doors are opened and materials are removed, both target samples and non-target (innocent) samples experience warming. However, when robotics are used to automatically locate and select samples within the cold storage unit, it creates a much more controlled environment for retrieval. A deeper understanding of the real effects caused by transient warming is imperative to minimize cellular damage and ensure effective treatments.
6. Ensure quality and compliance through data governance
Robust data management strategies are essential for quality and compliance. Along with the good manufacturing practice (GMP) compliance process, the FDA’s 21 CFR part 11 regulation ensures the integrity and security of electronic records and signatures as electronic documentation usage increases in clinical trials. Organizations regulated by the FDA must ensure processes are compliant, including audit trails for temperature monitoring with electronic time-stamps and date-stamps to demonstrate proper data governance and compliance.
7. Champion evolving standards
The establishment of industry standards for storage, materials and processes is central to success. Standards for cryopreservation are still evolving and that’s a big hurdle for further innovation. Clearly defined standards for microplates, for example, allow scientists to work with automated laboratory instrumentation across different labs and different equipment manufacturers. The SBS standard exists for cold storage at -20 and -80 degrees Celsius, but a similar standard does not exist for labware used at cryogenic temperatures. There is currently an industry-wide effort to create cell therapy cryopreservation standards and best practices, which was kicked off at the 2022 Cryogenic Considerations for Cell & Gene Therapy Symposium. Once these standards are in place, it will simplify and speed the work of cryobiologists to let them bring lifesaving therapies to market faster.
Summary
As the cell therapy landscape continues to expand, a grasp of these trends is indispensable. Over the past decade, scientists have made major advances using cryopreservation. Medical uses, particularly cell and gene therapy research and organ and tissue transplantation, are key drivers for this work. However, cryopreservation methods are often an afterthought for companies that work to develop innovative new therapies.
Proactive organizations are looking at what’s new in cryopreservation and automation to take advantage of how advances in cryo-technology can support their research to make it more productive and successful.
About the author
Erica Waller is a senior product manager for cryo automation and stores at Azenta Life Sciences, headquartered in Burlington, MA, USA. She holds a BS in Mechanical Engineering from MIT and was a systems engineer for Brooks Automation (now Azenta Life Sciences) for several years prior to her current role. Today, she oversees the cryo storage and automation portfolio for Azenta Life Sciences.