The Applications of Cell Therapy
In this article, we expand upon the applications of cell therapy, including its utility in cancer, regenerative medicine and autoimmunity.
Complete the form below to unlock access to ALL audio articles.
Contents
-CAR T-Cell Cancer Therapy
-Regenerative medicine
-Autoimmunity
Cell therapy: a recap
Cell therapy refers to the transplantation of human cells to either replace or repair damaged tissues and/or cells. A variety of different cell types, including stem cells and immune cells, are used as cell therapies. With an evolving understanding of our cells and the immune system, scientists are finding new ways to use our cells as therapeutic agents.
The uses of cell therapy
Below, we expand upon the applications of cell therapy, including its utility in cancer, regenerative medicine and autoimmunity.
CAR T-cell cancer therapy
Chimeric antigen receptor (CAR) T cell therapy is a cancer immunotherapy in which T cells are derived from a patient's blood and then genetically engineered in vitro to express an artificial receptor. This lets the T cells target specific tumor antigens.
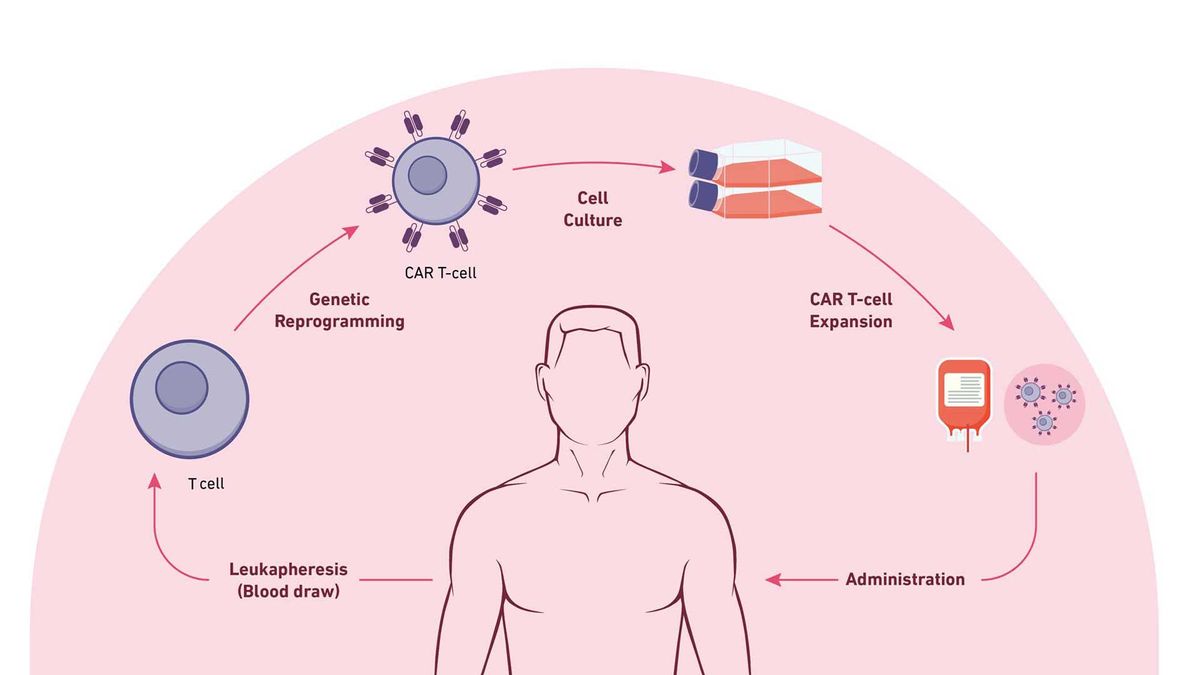
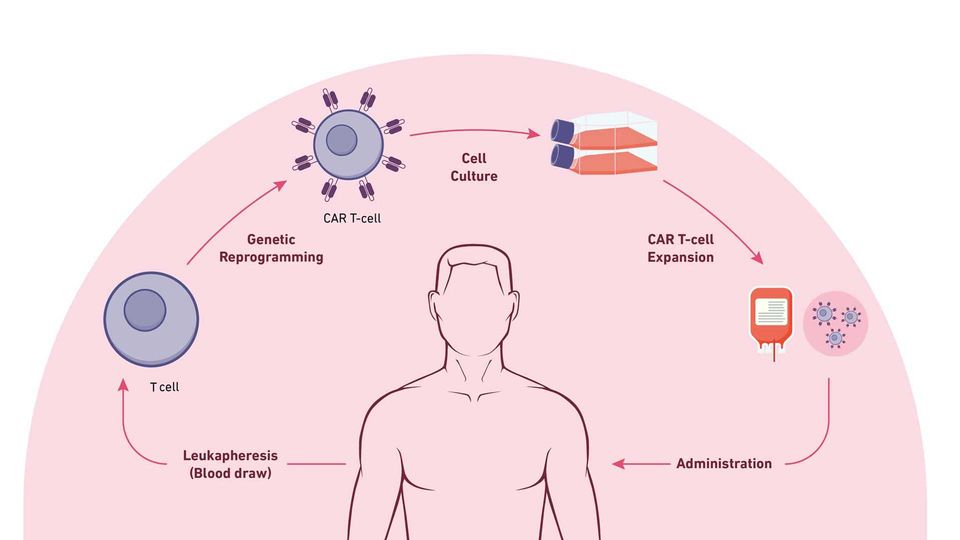
The steps of CAR T-cell therapy. Credit: Technology Networks
The steps involved for CAR T-cell therapy include:
Leukapheresis: Blood is drawn from the vein of the patient's arm; the blood moves through an apheresis machine in which the leukocytes are removed by continuous centrifugation, and the rest of the blood is returned to the patient.
T-Cell engineering and expansion: T cells are isolated from apheresis product and other cell types are removed that may inhibit T-cell expansion. The cells are then transduced with the CAR construct DNA using a viral vector, such as a lentivirus. The cells are checked to ensure they express the new receptor and then expanded until they reach an appropriate concentration (therapeutic dose).
CAR T-cell infusion: Before CAR T-cell infusion, the patient undergoes lymphodepletion (chemotherapy to remove lymphocytes), which allows a favorable immune environment for CAR T-cell expansion, persistence and therapeutic activity in vivo, once administered.
The engineered receptor is typically a mouse-derived monoclonal antibody presenting as a continuous peptide single-chain variable fragment (scFV) connected through an extracellular spacer domain to provide flexibility to the receptor.
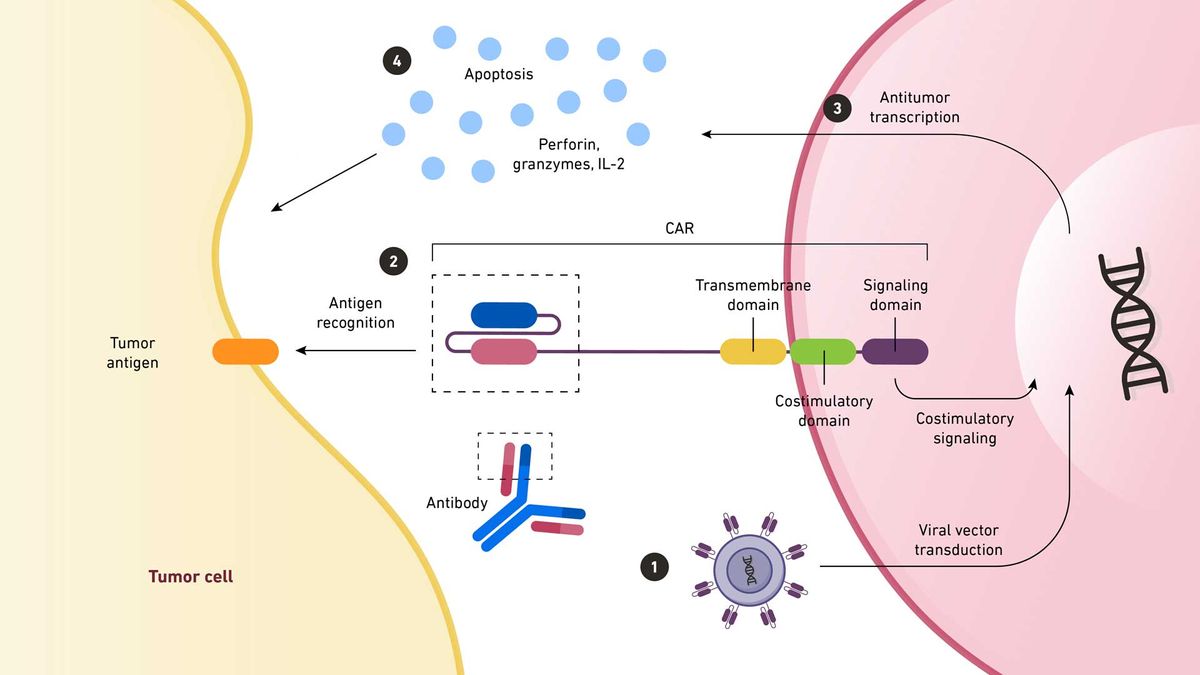
1. A viral vector transfers CAR-coding DNA into an immune cell. The antigen-binding region of the CAR is taken from a monoclonal antibody. 2. When this region recognizes an antigen on a tumor cell, the signal is amplified back to the nucleus. 3. Here, antitumor transcription begins. 4. This transcription produces cytokines that destroy the tumor cell. Credit: Technology Networks. Adapted from Chan et al. https://www.mdpi.com/2227-9059/10/4/804
CAR T-cell therapy has shown promise over the past decade, with 6 current FDA-approved products.
Regenerative medicine
Regenerative medicine has emerged as a new and promising therapeutic avenue for patients with limited or no other options for treatment. The main concept for regenerative medicine is tissue regeneration and replacing damaged cells. The use of stem cells has become an effective approach for treatment through the inhibition of inflammation and apoptosis. For example, stem cells can sense pro-inflammatory factors and reduce inflammation by releasing cytokines, such as TGFβ and IL-10, to inhibit dendritic cell migration, maturation, and antigen presentation. These factors can also suppress T-cell response.
Stem cells can also stimulate cell recruitment and angiogenesis and have the capability for differentiation.
The most popular cell types for regenerative medicine are hPSCs and mesenchymal/stromal stem cells. hPSCs are used for vision repair by differentiating into retinal pigment epithelium cells in the context of macular dystrophy.
MSCs have been found and derived from different sources, including:
- Adipose tissue (AT)
- Bone marrow (BM)
- Umbilical cord (UC)
- Placenta
- Amniotic fluid
- Dental pulp
The most popular MSCs used as therapeutics currently under clinical trial are derived from BM, UC and AT, with some already being FDA-approved. MSCs have been proven effective in treating multiple diseases, including those of the nervous system, pulmonary disease, cardiovascular conditions and wound healing.
These cells can be administered in many ways. Engineered materials are often used for administration; examples include cells encapsulated or coated with polymers or nanoparticles, scaffolds or films embedded with cells.
Autoimmunity
Autoimmunity refers to the human body mounting an attack against itself, producing a response against its cells, tissues, or other constituents, leading to chronic inflammation and injury. Typically, autoimmune diseases are related to an imbalance in the immune homeostasis. Examples of such conditions include rheumatoid arthritis (RA), type 1 diabetes, multiple sclerosis, lupus, and irritable bowel disease (IBD). Stem cells can be used to slow or stop the progression of autoimmune disease.
MSCs have been showed to be beneficial for autoimmune disease based on their immunosuppressive properties.
In inflammatory bowel disease, which includes diseases such as ulcerative colitis (UC) and Crohn's disease, the administration of MSCs showed therapeutic potential by restoring epithelial barrier integrity.
In the context of type 1 diabetes mellitus (T1DM), MSCs have been shown to play a role in lowering fasting blood sugar levels, hemoglobin A1c and C-peptide. Although multiple clinical trials are underway, the mechanism of action is still unknown.
Conclusion
Cell therapies showcase immense potential in revolutionizing medical treatments across various domains: The success of CAR T-cell therapy in treating certain cancers, regenerative medicine for tissue repair and treating autoimmune diseases.
CAR T and hematopoietic stem cell transplantation are the only FDA-approved cell therapies. However, almost 700 current stem cell-based clinical trials are taking place (almost 500 of these are based in the United States), according to the National Center for Biotechnology Information (NCBI), suggesting a bright future for the field.
References:
1. Fabrizio VA, Boelens JJ, Mauguen A, et al. Optimal fludarabine lymphodepletion is associated with improved outcomes after CAR T-cell therapy. In: Blood Advances. Vol 6. American Society of Hematology; 2022:1961-1968. doi:10.1182/bloodadvances.2021006418
2. Wagner DL, Fritsche E, Pulsipher MA, et al. Immunogenicity of CAR T cells in cancer therapy. Nat Rev Clin Oncol. 2021;18(6):379-393. doi:10.1038/s41571-021-00476-2
3. Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, Ganguly N. CAR T cell therapy: A new era for cancer treatment (Review). Oncol Rep. 2019;42(6):2183-2195. doi:10.3892/or.2019.7335
4. Office of Tissues and Advanced Therapies (OTAT). Approved Cellular and Gene Therapy Products. U.S. Food & Drug Administration. Published June 30, 2023. Accessed November 19, 2023. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
5. Shah NN, Lee DW, Yates B, et al. Long-Term Follow-Up of CD19-CAR T-Cell Therapy in Children and Young Adults With B-ALL. J Clin Oncol. 2021;39:1650-1659. doi:10.1200/JCO.20
6. Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20(6):359-371. doi:10.1038/s41571-023-00754-1
7. Salari V, Mengoni F, Gallo F Del, Bertini G, Fabene PF. The anti-inflammatory properties of mesenchymal stem cells in epilepsy: Possible treatments and future perspectives. Int J Mol Sci. 2020;21(24):1-15. doi:10.3390/ijms21249683
8. Mousaei Ghasroldasht M, Seok J, Park HS, Liakath Ali FB, Al-Hendy A. Stem Cell Therapy: From Idea to Clinical Practice. Int J Mol Sci. 2022;23(5). doi:10.3390/ijms23052850
9. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. www.thelancet.com. 2012;379:713. doi:10.1016/S0140-6736(12)60028-2
10. Limnios IJ, Chau YQ, Skabo SJ, Surrao DC, O’Neill HC. Efficient differentiation of human embryonic stem cells to retinal pigment epithelium under defined conditions. Stem Cell Res Ther. 2021;12(1). doi:10.1186/s13287-021-02316-7
11. Menasché P, Vanneaux V, Hagège A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur Heart J. 2015;36(30):2011-2017. doi:10.1093/eurheartj/ehv189
12. McKenna SL, Ehsanian R, Liu CY, et al. Ten-year safety of pluripotent stem cell transplantation in acute thoracic spinal cord injury. J Neurosurg Spine. 2022;37(3):321-330. doi:10.3171/2021.12.SPINE21622
13. Hoang DM, Pham PT, Bach TQ, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7(1). doi:10.1038/s41392-022-01134-4
14. Yu Y, Wang Q, Wang C, Shang L. Living Materials for Regenerative Medicine. Engineered Regeneration. 2021;2:96-104. doi:10.1016/j.engreg.2021.08.003
15. Chen S, Hsieh MH, Li SH, et al. A conductive cell-delivery construct as a bioengineered patch that can improve electrical propagation and synchronize cardiomyocyte contraction for heart repair. Journal of Controlled Release. 2020;320:73-82. doi:10.1016/j.jconrel.2020.01.027
16. Koivusalo L, Kauppila M, Samanta S, et al. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials. 2019;225. doi:10.1016/j.biomaterials.2019.119516
17. Jasim SA, Yumashev AV, Abdelbasset WK, et al. Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases. Stem Cell Res Ther. 2022;13(1). doi:10.1186/s13287-022-02782-7
18. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Published online 2005. doi:10.1182/blood-2004-04-1559
19. Barnhoorn MC, Wasser MNJM, Roelofs H, et al. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J Crohns Colitis. 2020;14(1):64-70. doi:10.1093/ecco-jcc/jjz116
20. Jayasinghe M, Prathiraja O, Perera PB, et al. The Role of Mesenchymal Stem Cells in the Treatment of Type 1 Diabetes. Cureus. Published online July 27, 2022. doi:10.7759/cureus.27337
21. National Library of Medicine. Clinical Trials, Stem Cell Therapy. Accessed January 6, 2024. https://clinicaltrials.gov/search?term=Stem%20Cell%20Therapy&aggFilters=status:act



