Applied Biosystems VeritiPro Dx: IVDR-Compliant, CE-IVD Labeled Diagnostic Thermal Cycler
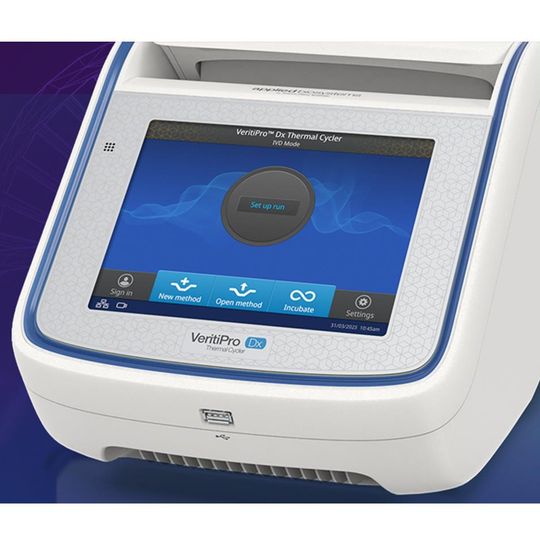
The Applied Biosystems VeritiPro Dx Thermal Cycler is an in vitro diagnostic (IVD) endpoint thermal cycler designed to amplify nucleic acids from human-derived specimens using polymerase chain reaction (PCR). It is manufactured to ISO 13485 and GMP requirements.
VeritiPro Dx Thermal Cycler conforms to:
- EU 2017/746 requirements and is CE- IVD labeled in Europe
- Classified as US-FDA class I medical device
- UK Medical devices regulations 2002 and is UKCA labeled in UK
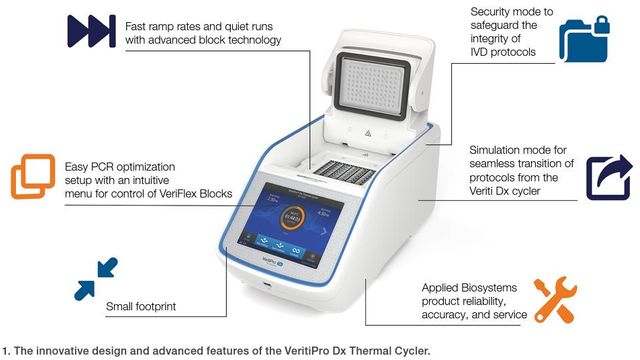
This instrument delivers precise temperature control with a ramp rate of 6.0°C/sec. There is no need for protocol re-optimization when upgrading from Veriti Dx Thermal Cycler; the simulation mode supports seamless transition