Organoids are primary patient-derived micro tissues grown within a three-dimensional extracellular matrix. They offer a better representation of in vivo physiology than existing two-dimensional cell lines; however, organoid culturing requires additional considerations and tissue-specific formulations that may not be available commercially.
This guide equips researchers with a standardized approach for routine handling of organoids, including protocols that can be applied to both normal and diseased tissue from various tissue types.
Download this guide to learn more about:
- How to grow and expand organoids to study cells with complex organization and disease-state phenotypes
- Cryopreservation of organoids and how to thaw them
- Critical parameters for success and how to troubleshoot common problems
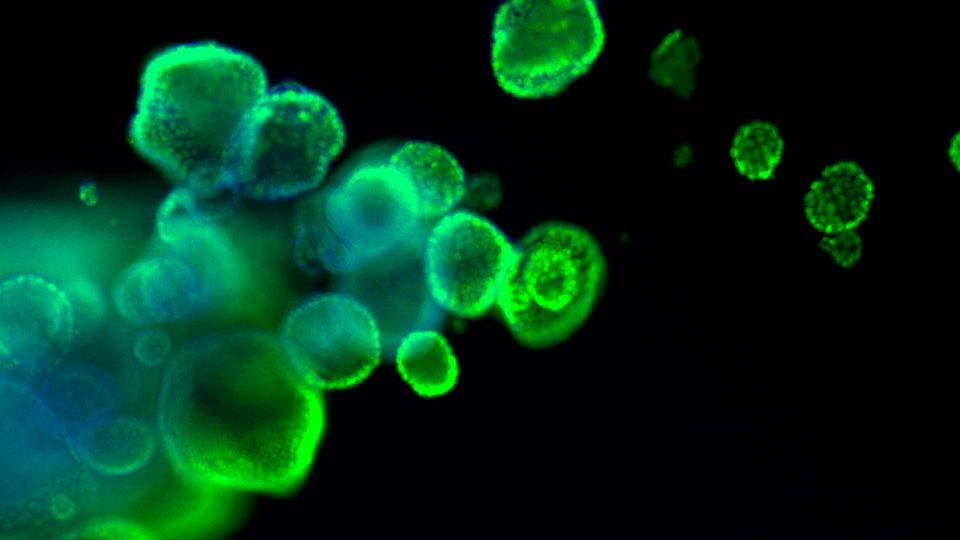
Organoids are primary patient-derived micro tissues grown within a three dimensional extracellular matrix that better represents in vivo physiology and genetic diversity than existing two-dimensional cell lines. Organoids rely on the self-renewal and differentiation of tissue-resident stem cells that expand in culture and self-organize into complex three-dimensional structures. Depending on the tissue, organoids typically lack stromal, vascular, neural, and immune cells but otherwise can contain cells from all the respective tissue-specific cell lineages found in vivo. Established organoids can be initiated from cryopreserved material, cultured using largely traditional cell culture techniques and equipment, and then expanded and cryopreserved for future use. Organoid models have been developed from a variety of diseased and normal tissues including small intestine, colon, mammary, esophagus, lung, prostate, and pancreas. INTRODUCTION This article describes protocols for the three-dimensional (3-D) in vitro culture of human primary tissue-derived organoids starting from cryopreserved material. Organoids are self-organizing, self-renewing micro tissues derived from stem cells isolated from normal or diseased samples such as tumor resections or needle biopsies. Organoid technology permits the expansion of cells that would otherwise not proliferate in culture, and simultaneously maintains in vivo-like characteristics such as complex organization, tissue-specific functions, and disease-state phenotypes. Single cells or fragments from primary tissues are suspended in 3-D within an undefined extracellular matrix (ECM) derived from Engelbreth-Holm-Swarm (EHS) murine sarcoma, which takes the form of a gel dome spotted onto standard tissue-culture-treated plastic. Once solidified, these domes are overlaid with a complex medium formulation containing small molecules, recombinant proteins, and other supplements that may be tissue and disease specific. Figure 1: Embedded 3-D “dome” organoid culture workflow. Individual cells or organoid fragments are embedded within a liquid extracellular matrix (ECM) and dispensed as small droplets onto the surface of a warm tissue culture plastic vessel. The ECM will solidify into a gel after incubation at 37°C and can then be covered with culture medium. Organoids will develop within the dome as 3-D structures. Over time in culture, the cells or fragments will expand in number and self-organize into 3-D structures (see Figure 1). Organoids can be propagated and expanded by removal of the ECM followed by enzymatic and/or mechanical dissociation. The dissociated organoids are then returned to 3-D culture conditions to continue expansion and subsequently re-develop into organoids. Organoids are amenable to many standard in vitro assays including RNA and DNA isolation, immunohistochemistry, and genetic manipulation. HOW TO CITE THIS ARTICLE: Clinton, J., & McWilliams-Koeppen, P. (2019). Initiation, expansion, and cryopreservation of human primary tissue-derived normal and diseased organoids in embedded three-dimensional culture. Current Protocols in Cell Biology, 82, e66. doi: 10.1002/cpcb.66Page 3 Order online at www.atcc.org, call 800.638.6597, 703.365.2700, or contact your local distributor. This article presents a standardized approach for routine handling of organoids that can be applied to both normal and diseased tissue from various tissue types. The basic steps involved will be familiar to those with experience culturing more traditional two-dimensional cultures of continuous or primary cells. However, organoid culture requires additional considerations and preparation stemming from complex, tissue-specific medium formulations that may not be available commercially, difficulty in counting and manipulating precise cell numbers, the use of multiple undefined components subject to batch-to-batch variation, and a 3-D culture format that utilizes various extracellular matrices with unique handling requirements. Basic Protocol 1 describes the steps to initiate organoid culture from cryopreserved material. Basic Protocol 2 describes maintaining and expanding organoid cultures. Basic Protocol 3 describes how to cryopreserve organoids. Note that the basic protocols below are written for a hypothetical standard vial of cryopreserved organoids that does not represent any specific model, tissue, or disease. Refer to any model-specific details p